PATENT
“Selective Anti-Cancer Agent Effective
For Prevention and Treatment”
Granted US Patent Published August 10, 2021 (US11083714B2)
Granted European Patent Published October 25, 2023 (EP3773582B1)
Granted Japanese Patent Published May 20, 2024 (7491314)

Three Key Patent Claims
Anti-Cancer
Anti-Cancer
1. A therapeutic method comprising administering a de-ethylflavopereirine compound (N13-9-1) in one or more doses to a subject afflicted with or at elevated risk for cancer….
Drug Combinations
Drug Combinations
15. The method, further comprising co-administering simultaneously or sequentially an effective amount of a chemotherapeutic agent other than the compound, wherein the chemotherapeutic agent is also active in the treatment of cancer.
Anti-Inflammation
Anti-Inflammation
13. A method of treating inflammation and preventing resultant tissue damage comprising administering in one or more doses to a subject in need thereof a de-ethylflavopereirine compound…in an amount effective to reduce inflammation and thereby prevent tissue damage by infiltrating leukocytes.
Full Claim SET of U.S. Patent 11,083,714 B2
- A therapeutic method comprising administering a de-ethylflavopereirine compound in one or more doses to a subject afflicted with or at elevated risk for cancer; wherein the compound is de-ethylflavopereirine, a salt thereof, a solvate thereof, a hydrate thereof, or a salt of the solvate or the hydrate.
- A method for treating a subject afflicted with cancer or chronic inflammation; the method comprising administering to the subject a pharmaceutical composition or a medical device; wherein the pharmaceutical composition or the medical device comprises a de-ethylflavopereirine compound selected from the group consisting of de-ethylflavopereirine, salts of de-ethylflavopereirine, solvates of de-ethylflavopereirine, salts of the solvates, hydrates of de-ethylflavopereirine, and salts of the hydrates.
- A pharmaceutical composition or a medical device, including a kit of parts, manufactured by a process comprising incorporating at least one de-ethylflavopereirine compound selected from the group consisting of de-ethylflavopereirine, salts thereof, solvates thereof, hydrates thereof, salts of the solvates, and salts of the hydrates in the pharmaceutical composition or the medical device.
- The method according to Claim 2, wherein the pharmaceutical composition is administered systemically.
- The method according to Claim 2, wherein the pharmaceutical composition is administered locally.
- The method according to Claim 2, wherein the pharmaceutical composition is administered at least enterally.
- The method according to Claim 2, wherein the pharmaceutical composition is administered at least parenterally.
- The method according to Claim 2, wherein the pharmaceutical composition is formulated for at least topical application, inhalation, ophthalmic administration, or sublingual administration.
- The method according to Claim 2, wherein the medical device is implantable.
- The method according to Claim 2, further comprising administering an agent other than a de-ethylflavopereirine compound, wherein the agent is active in the treatment of cancer and is administered by the same or a different route as the de-ethylflavopereirine compound.
- The method according to Claim 1, wherein the cancer is characterized as at least one disease selected from the group consisting of carcinomas, sarcomas, melanomas, leukemias, and lymphomas.
- The method according to Claim 1, wherein the cancer is selected from the group consisting of lung, spleen, colon, kidney, liver, pancreatic, and ovarian cancers.
- A method of treating inflammation and preventing resultant tissue damage comprising administering in one or more doses to a subject in need thereof a de-ethylflavopereirine compound selected from the group consisting of de-ethylflavopereirine, salts of de-ethylflavopereirine, solvates of de-ethylflavopereirine, salts of the solvates, hydrates of de-ethylflavopereirine, and salts of the hydrates in an amount effective to reduce inflammation and thereby prevent tissue damage by infiltrating leukocytes.
- A therapeutic method comprising administering perorally an effective amount of a compound in solid form and packaged into one or more unit doses to a human patient afflicted with cancer to eliminate cells of a malignant tumor and/or metastasis; wherein the compound is selected from the group consisting of de-ethylflavopereirine, salts of de-ethylflavopereirine, solvates of de-ethylflavopereirine, salts of the solvates, hydrates of de-ethylflavopereirine, and salts of the hydrates.
- The method according to Claim 14, further comprising co-administering simultaneously or sequentially an effective amount of a chemotherapeutic agent other than the compound, wherein the chemotherapeutic agent is also active in the treatment of cancer.
- The method according to Claim 14, wherein the cancer cells eliminated include a cancer stem cell.
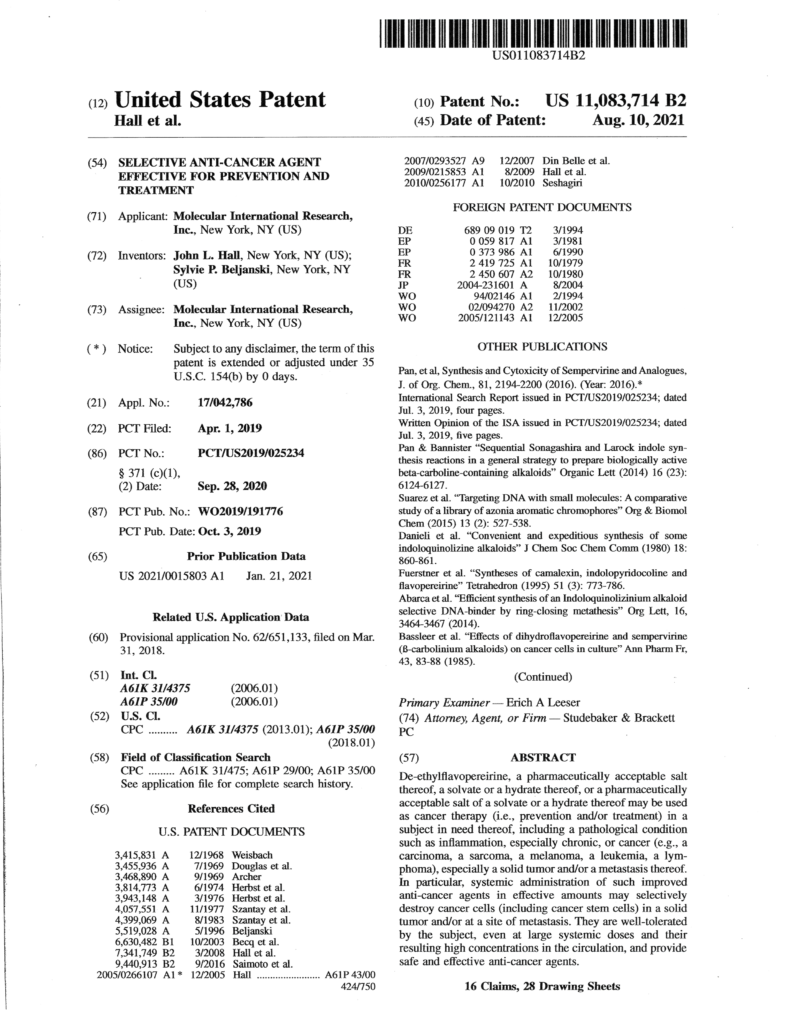
US Patent 11,083,714B2 (‘714) Valuation
Broad spectrum and selective action of N13-9-1 support extensive applications in oncology, as well as an important contribution to treatment of inflammation.
Lung cancer is the first application in our valuation with the highest Fair Market Value reflecting, in part, greater incidence. Treatment of pancreatic cancer for which novel therapy is also urgently needed is the second application and Inflammation is the third application. Conservative assumptions (e.g. 15% market share) are behind the Fair Market Value which totals approximately $800 million.
*This valuation is a forward-looking statement under Federal Securities Law and there is no assurance that it will prove to be accurate. It assumes that MPI is a going concern with FDA approval. Actual value may be higher or lower. The validity of evaluation depends on the assumptions stated in the evaluation, which merit close study by potential investors
*This US ‘714 Patent Valuation does not include the value of the EU Patent.
