Anti-cancer
Based on cell and animal studies, it appears that our drug candidate, N13-9-1, selectively targets tumor cells without toxicity to healthy cells and organs: efficacy and safety co-exist. Our scientists expect that:
- N13-9-1 will work against most types of cancer and promise to be effective against the most difficult to treat; colon, lung, and pancreatic cancers.
- N13-9-1 restores chemosensitivity to other drugs in combination and is active against cancer stem cells.
- N13-9-1 also functions as a regulator of inflammatory response, potentially opening a second set of applications and markets.
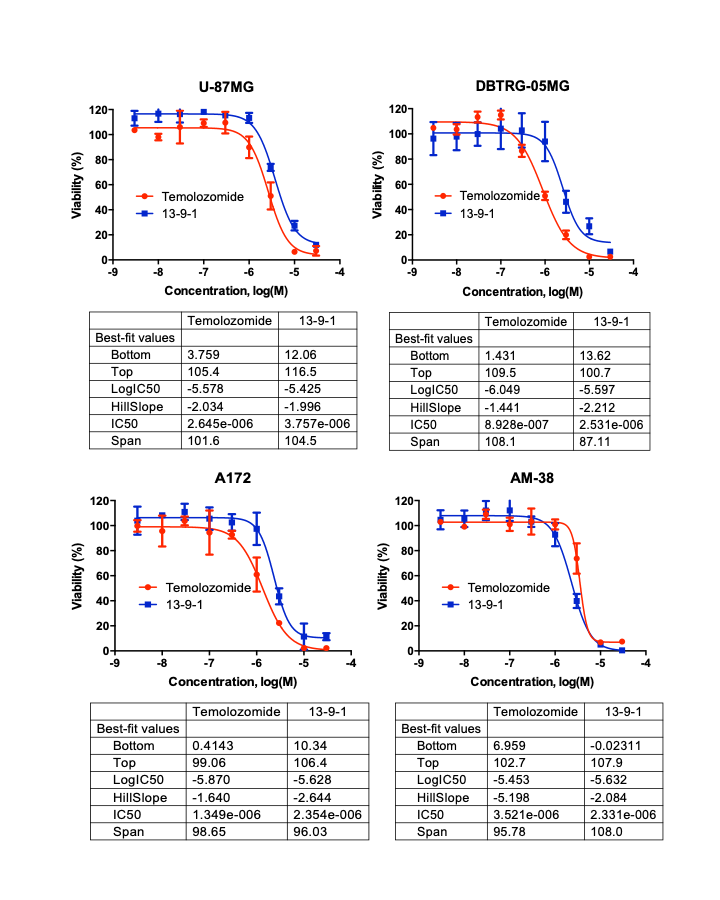
N13-9-1 for brain cancer: same anti-cancer effect as mainstream drug, but with selectivity for tumor cells
Our new patent presents the details of the chemical synthesis of N13-9-1 as well as confirmation of the structure and purity of the synthetic product. For the sulfate salt, solubility in water is excellent and anti-cancer effect is demonstrated, so this salt was selected for ongoing experiments. Our data show activity against brain, breast, colon, lung, ovarian, pancreatic, and prostate cancer cells.
N13-9-1 is effective against most cancers—a clinically significant advantage that enhances the value of our patent. In the case of glioblastoma (brain cancer), the dose response curves for N13-9-1 and Temolozomide, a drug commonly used for this cancer, are very similar: low micromolar effective concentrations and similar IC50 values.
In cell-based assays, N13-9-1 acts like a mainstream anti-cancer drug in terms of killing cancer cells, the critical difference is its selectivity for cancer cells.
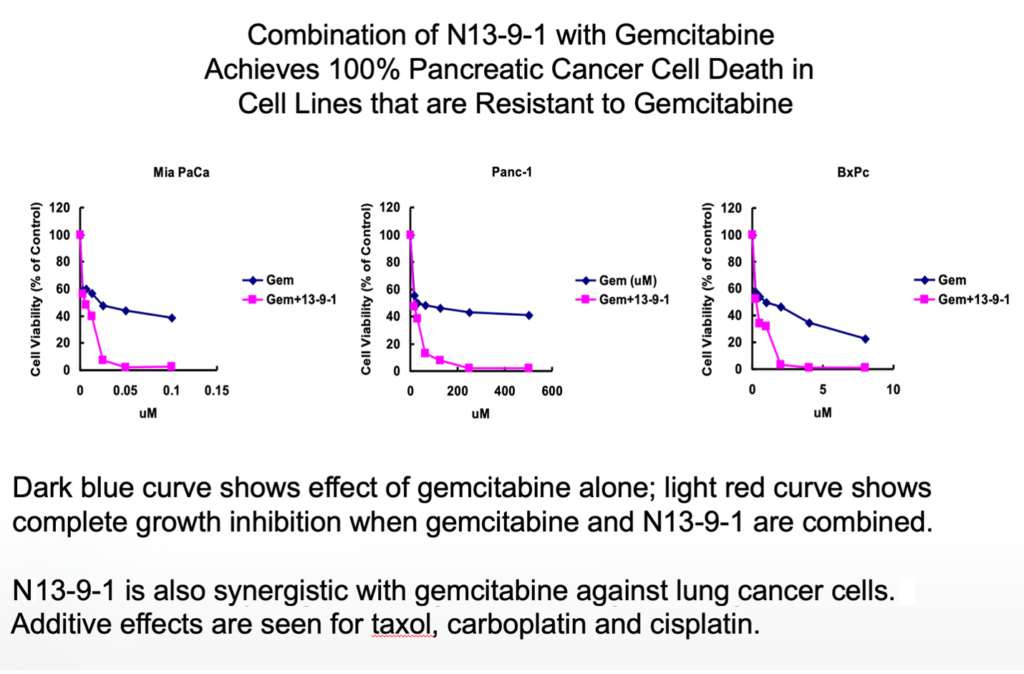
Combinations with other drugs and therapies
Cell-based assays showed that N13-9-1 works by itself to knock off cancer cells, but also combines very well with other anti-cancer drugs. Tested against pancreatic or lung cancer, combinations with carboplatin or taxol are multiplicative, combinations with gemcitabine are synergistic.
We expect N13-9-1 to work with immunotherapies and targeted therapies because it’s novel mechanism of action (targeting the damaged DNA in cancer cells) is unlikely to conflict with the operation of these newer types of therapy.
Animal studies – tumor reduction with NO apparent toxicity
In animal studies, we have established activity against colon cancer and pancreatic cancer with no evidence of toxicity in the animals: tumors shrink, and no negative side effects are detected. Several observations support this safety profile: 1) animals continue to grow and gain weight normally; 2) the anatomy of internal organs remains normal; and 3) the behavior of the animals is normal in terms of their orientation and level of activity.
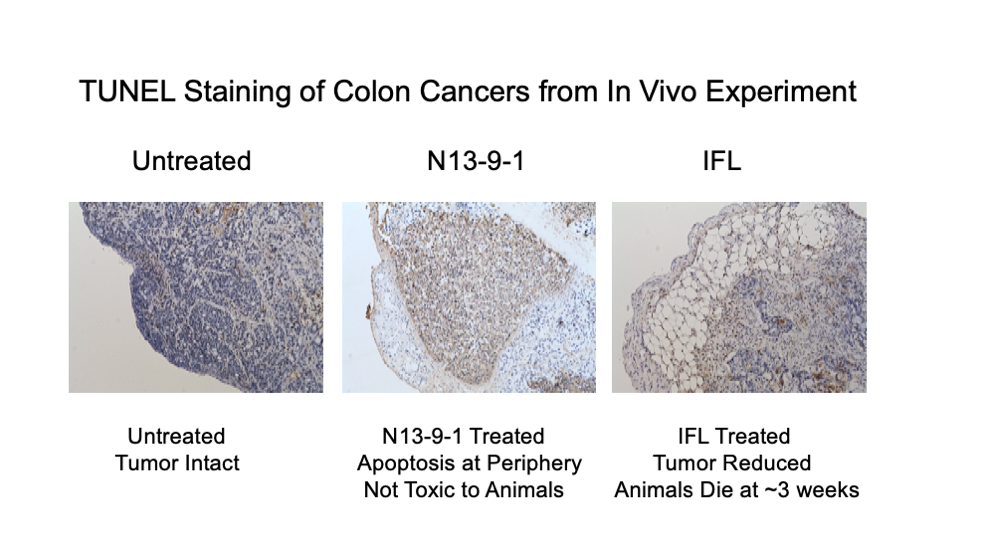
In our colon cancer experiment, IFL was used as a control, and we encountered the toxicity associated with this drug cocktail which has a history of use for colon cancer. IFL is a combination of 3 aggressive drugs: irinotecan, fluorouracil and leucovorin. In fact, this experiment had to be terminated after 3 weeks because the animals in the IFL treatment group started to die. The animals in the N13-9-1 group were fine and evidence of tumor reduction was shown by TUNEL staining which detects the process of apoptosis or cancer cell death. N13-9-1 was used at concentrations of 20mg/kg (colon cancer) and 100mg/kg (pancreatic cancer) and it is clear from other dose finding experiments that higher doses (300mg/kg) are well tolerated. What’s more, oral administration is the favored route of delivery. This is a major benefit of an anti-cancer and anti-inflammatory drug in terms of patient quality of life, convenience, and cost of care.
Cancer stem cells
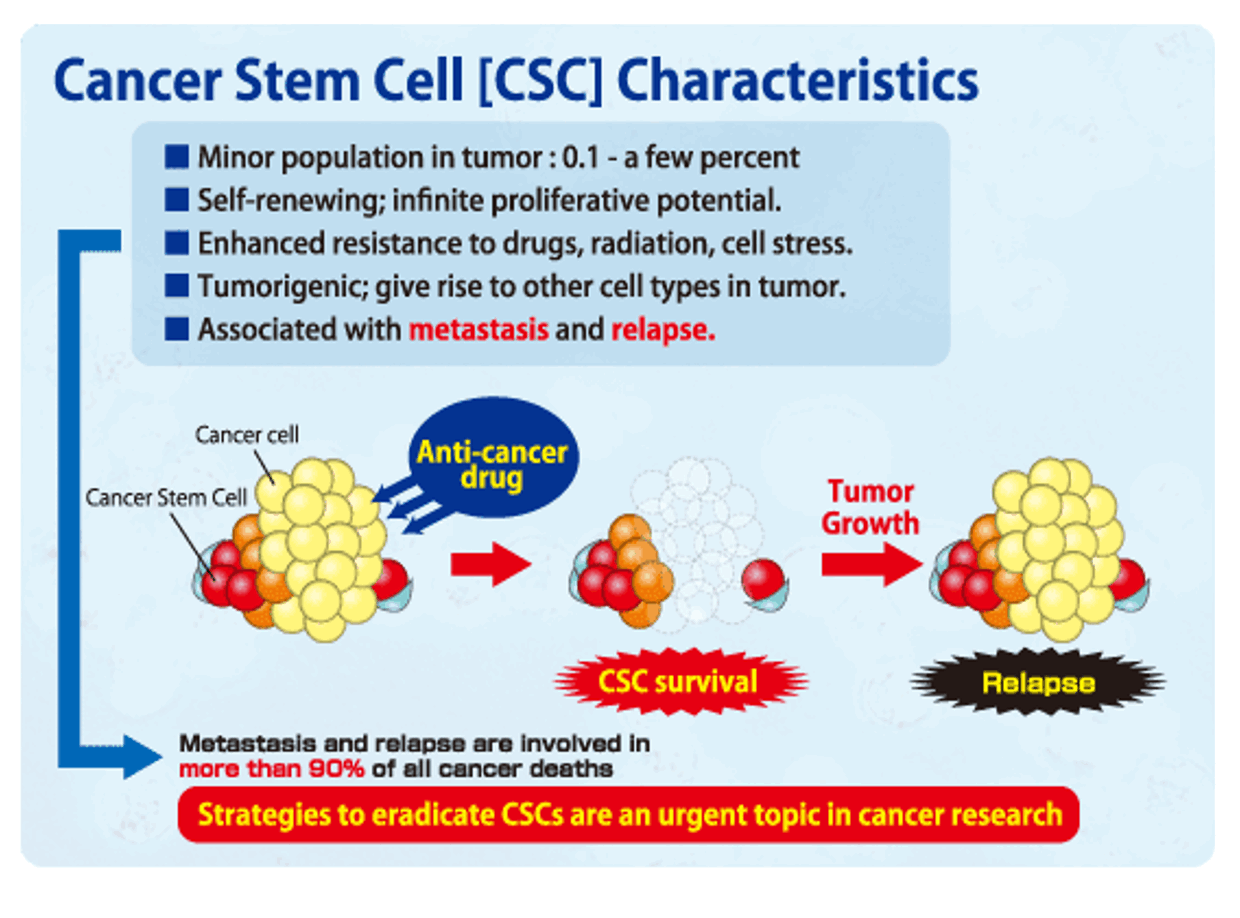
Cancer stem cells (CSCs) are a subpopulation of the cells in a tumor that are responsible for the recurrence of cancer that often occurs following treatment and remission. Cancer stem cells are so-called because they share key characteristics with healthy stem cells including the potential for persistent survival and self-renewal. CSCs have also proved to be resistant to anti-cancer drugs which enables survival throughout the course of cancer treatments. At some point after remission, the cancer stem cells migrate to other sites in the body and begin actively dividing—meaning that the cancer has returned and metastasized. Clearly, drugs that attack cancer stem cells are a high priority for future cancer therapies. Our experiments show that N13-9-1 attacks cancer stem cells.
N13-9-1 attacks cancer stem cells
We found that N13-9-1 significantly reduces the number of cancer stem cells (identified by three cell surface markers) in the total population of a human pancreatic cancer cell line.
N13-9-1 also limits the defining activity of these pancreatic cancer stem cells: the formation and growth of new tumors. Cancer stem cell data are shown in the patent.
